The Best Practice Surgical and Refractive Management of Cataract (BRIGhT): Randomised Controlled Trial will investigate the impact on falls risk & quality of life, as well as cost-effectiveness of expedited surgery & refractive correction for patients with bilateral cataract. Currently, a pilot study investigating the feasibility of implementing the BRIGhT randomised controlled trial is being conducted at two major public hospital sites.
Details of the pilot study are listed on the Australian New Zealand Clinical Trials Registry website.
Background
Cataract is a leading cause of vision impairment globally. Around 1.2 million Australians over the age of 65 have clinically significant cataract, which places them at a risk of falling that’s three times higher than normal.1,2 In Australia, falls cost over $1 billion in treatment, disability, lost output and mortality each year.3
While cataract surgery is highly effective at restoring sight, long waiting times for cataract surgery in the public hospital system are common in Australia.4,5 Patients can wait up to three years for their first eye surgery: an initial two years wait for an outpatient ophthalmology assessment and a further 12 months on the surgical waiting list.5,6
There is evidence that reducing wait times for cataract surgery can reduce the rate of falls among people over 65 years of age.7,8 A study in the United Kingdom found a 34% reduction in falls of patients waiting for cataract surgery, by reducing surgery wait time from 12 months to one month.7
In Australia, the FOCUS Study (Falls in Older people with Cataract, a longitudinal evaluation of impact and risk) followed patients aged over 65 years with bilateral cataract as they waited for surgery. Of the 329 participants, 31% experienced a fall while waiting for first eye cataract surgery.8 Over one half of falls (52%) caused injury, including 14 head injuries and two fractures. It was estimated that 91 falls could potentially be prevented, if participants received cataract surgery within one month of referral, compared to their actual wait time.7,8
Based on these findings, the Best Practice Surgical and Refractive Management of Cataract (BRIGhT): Randomised Controlled Trial was designed to investigate the impact of expediting cataract surgery on patient outcomes and also cost-effectiveness.
Aim
To explore the impact of a combined intervention of expedited, sequential cataract surgery and refractive correction on falls risk and quality of life in older Australians.
Benefits
- This pilot study will determine the feasibility of the BRIGhT Study with respect to recruitment rate, adherence to study protocol, scalability, cost and other issues relevant to implementation.
- In the future, full-scale implementation of the BRIGhT Study aims to uncover the impact of and cost of delays to cataract surgery on falls and quality of life of older people with cataract. These findings could support initiatives to reduce wait times for cataract surgery through policy development and improved resourcing for cataract surgical services.
Implementation
Currently, the feasibility of implementing a large scale randomised controlled trial is being investigated in a pilot study of 40 participants. Details of the study are provided below.
Participants
- Participants awaiting cataract surgery will be recruited from eye clinics at two public hospitals in Sydney. The following eligibility criteria applied to all participants in the study:
- aged 65 years or older
- bilateral cataract, presenting for their first eye cataract surgery (no combined surgery)
- no diagnosis of dementia, Parkinson’s disease or stroke
- living in the community or a self-care unit of a retirement village
- no other significant ocular comorbidities
- able to walk (not wheelchair bound)
- able to complete surveys and falls calendars as required (see: Data Collection below).
Study design
Potentially eligible participants will be invited to the study initially via a letter then follow-up telephone call (Figure 1) . Subsequent to providing informed consent, a baseline assessment will be performed and participants will be randomised to receive either the intervention (expedited, sequential cataract surgery and refractive correction) or usual care and followed for 12 months.
Figure 1: A flowchart of the BRIGhT study design
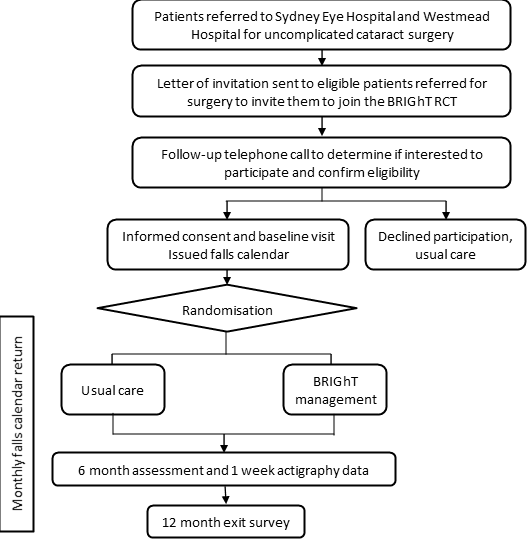
Data collection
- At baseline and six month follow-up visits, the following data will be collected:
- measures of visual function (visual acuity, contrast sensitivity, stereopsis, refractive error, spectacle correction)
- visual disability (Catquest-9SF)
- medications and comorbidities
- history of previous falls
- depressive symptoms (5-item Geriatric Depression Scale) and quality of life (EQ-5D-5L)
- exercise frequency (Incidental and Planned Exercise Questionnaire).
- Over the 12-month study period, falls will be self-reported by participants using a monthly calendar.
- Any falls will be followed up by a telephone call from the research team to determine fall circumstances, injuries sustained, and treatment received.
- At the six month follow-up, participants will be provided with an activity monitor for one week to allow estimation of physical activity.
- At the end of the study, cataract surgery details will be collected from hospital medical records.
Project status
Implementation – The project is ready for implementation or is currently being implemented, piloted or tested.
Implementation sites
Sydney Eye and Westmead Hospitals.
Partnerships
- National Health and Medical Research Council
- NSW Agency for Clinical Innovation
- Save Sight Institute, University of Sydney
- The George Institute for Global Health, UNSW Australia
- Westmead Institute for Medical Research, Westmead Hospital
References
- Australian Institute of Health and Welfare (AIHW). Vision problems among older Australians. Canberra: AIHW; 2005.
- McCarty CA, Fu CL, Taylor HR. Predictors of falls in the Melbourne visual impairment project. Australian and New Zealand Journal of Public Health. 2002;26(2):116-119.
- Moller J. Cost of injury. National estimates based on the cost model reported by Watson and Ozanne-Smith. Canberra: AIHW; 1998.
- Lansingh VC, Carter MJ, Martens M. Global cost-effectiveness of cataract surgery. Ophthalmology. 2007;114(9):1670-78.
- AIHW. Australian hospital statistics 2013-14: elective surgery waiting times. Canberra: AIHW; 2014.
- Victorian Government Department of Human Services. Victorian ophthalmology services planning framework. Melbourne: State Government Victoria; 2005.
- Harwood RH, Foss AJ, Osborn F et al. Falls and health status in elderly women following first eye cataract surgery: a randomised controlled trial. British Journal of Ophthalmology. 2005;89(1):53-59.
- Palagyi A, McCluskey P, White A, et al. While We Waited: Incidence and Predictors of Falls in Older Adults with Cataract. Investigative Ophthalmology Vision Science. 2016;57(14):6003-6010.
Further reading
- Palagyi A, McCluskey P, White A, et al. While we waited: incidence and predictors of falls in older adults with cataract. Investigative Ophthalmology & Visual Science, 2016;57:6003-6010.
Contact
Lisa Keay
Deputy Director, Injury Division
The George Institute for Global Health, UNSW Australia
Phone: 02 8052 4335
lkeay@georgeinstitute.org.au